CIDP. Patients with this rare disease have gained another dose. "The patient's life is definitely changing."

- In patients with CIDP who do not respond to corticosteroid therapy, treatment may be provided under drug program B.67
- The drug therapy program involves administering immunoglobulins via intravenous infusions, which requires hospitalization of the patient, usually once a month.
- Once CIDP has stabilized, subcutaneous immunoglobulins can be administered.
- Access to subcutaneous immunoglobulins significantly improves the comfort of treatment. It enables a transition from multi-hour intravenous infusions in a hospital setting to home administration, emphasizes Dr. Anna Potulska-Chromik from the Department and Clinic of Neurology at the Medical University of Warsaw.
- From July 1, patients have been provided with another therapeutic option in this regard.
- The ability to self-administer the drug at home is very important for CIDP patients, as it is a chronic disease that requires lifelong treatment - emphasizes Lucyna Wierzowiecka, president of axON - Association for Patients with CIDP, GBS, MMN
Chronic inflammatory demyelinating polyneuropathy ( CIDP ) is a rare autoimmune neurological disease in which the immune system attacks the myelin sheaths of peripheral nerves .
This leads to impaired nerve conduction, resulting in muscle weakness (especially in the lower and upper limbs), sensory disturbances, tingling, numbness, and difficulty moving. The disease develops gradually, usually over several weeks or months, and may be progressive or relapsing-remitting.
It is estimated that chronic inflammatory demyelinating polyneuropathy affects approximately 0.004-0.007% of the population, so it can be assumed that in Poland 2-2.6 thousand people suffer from CIDIP.
CIDP treatment can be implemented under the B.67 drug program for the treatment of neurological diseases with immunoglobulins.
As of July 1, the Ministry of Health has introduced a second subcutaneous immunoglobulin preparation into the program – subcutaneous immunoglobulin with hyaluronidase, which aims to improve access to home therapy and the quality of life of patients with CIDP.
Self-administration of subcutaneous immunoglobulin allows CIDP patients to avoid frequent hospitalizations required when treated with intravenous immunoglobulins.
"The ability to self-administer immunoglobulin subcutaneously at home significantly changes a patient's life. It allows them to return to normal functioning at work and at home, without the need for regular, usually monthly, hospitalizations. This comfort is extremely important in CIDP, which is a chronic disease requiring lifelong treatment. We are pleased and grateful that the Ministry of Health has recognized the needs of CIDP patients and is introducing additional modern medications for reimbursement," says Lucyna Wierzowiecka , president of the axON Association for Patients with CIDP, GBS, and MMN .
Subcutaneous immunoglobulins improved the quality of life of patients with CIDPStandard treatment for CIDP involves the administration of corticosteroids. In approximately 40-50% of CIDP patients, corticosteroid therapy is ineffective or contraindicated. These patients qualify for intravenous immunoglobulin therapy (IVIg). Some patients also receive immunosuppressive medications or plasmapheresis.
In the B.67 drug program, CIDP patients receive intravenous immunoglobulins. The program also offers the option of administering subcutaneous immunoglobulins once the disease has stabilized. Immunoglobulins are administered via infusion pumps.
Immunoglobulin therapy aims to modulate the immune response and alleviate neurological symptoms. It can improve patients' quality of life and slow disease progression.
Maintaining disease stabilization achieved with intravenous administration of immunoglobulin preparations has become easier for patients thanks to reimbursed access to subcutaneous immunoglobulins under the B.67 program.
"Once stabilized in CIDP, training the patient to self-administer subcutaneous immunoglobulin may be considered. Access to subcutaneous immunoglobulins significantly improves the comfort of treatment. It allows for a transition from multi-hour intravenous infusions in a hospital setting to subcutaneous administration at home," emphasizes Dr. Anna Potulska-Chromik, noting that this is particularly important for students and workers.
Two years ago, the Ministry of Health introduced conventional subcutaneous immunoglobulin (SCIG) into the B.67 drug program. At that time, patients were able to self-administer the immunoglobulin at home for the first time. The drug is required to be administered every two weeks.
Another change to the B.67 program, introduced on July 1, 2025, involves the addition of a new form of subcutaneous immunoglobulin—fSCIG (HyQvia with hyaluronidase). This expands therapeutic options, which is important for the diverse course of CIDP. This form of immunoglobulin is administered every 3-4 weeks.
It is characterized by high bioavailability (comparable to intravenous immunoglobulins), and clinical studies have confirmed its high effectiveness and safety.
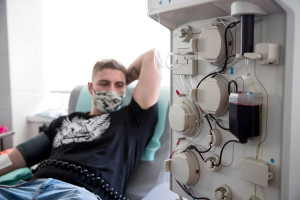
3-month supply of the drugThe administration of subcutaneous immunoglobulins is less burdensome for the patient than intravenous administration, because the infusion usually lasts 1-2 hours and does not require hospitalization.
"I've returned to a full life. I function like a healthy person: I run, and the disease doesn't limit me at work. Previously, when going to meetings, I would check if there was an elevator in the building, often having to use crutches," says Ernest Syska, a CIDP patient who runs his own business and has switched to subcutaneous immunoglobulin.
"For intravenous administration, you need to get to a specialized center and spend a minimum of six hours there. Sometimes, administering the medication requires staying in the hospital until the next day, which means a two-day absence from work and home," he shares his experience with previous treatment.
- Now that I administer immunoglobulin myself, subcutaneously, I go to the hospital for a consultation once every three months, because I receive a supply of the drug to take home for that period - he explains.
He also highlights another important aspect, the possibility of subcutaneous self-administration. In patients treated with intravenous immunoglobulin infusions, the frequency of drug administration is scheduled based on the patient's needs and the capabilities of the center providing specialized treatment.
In the case of patients using subcutaneous immunoglobulins on their own, it is easier in some cases to achieve better symptom control, because the drug, after consultation with the doctor, can be taken, for example, in smaller doses but more frequently.
"When intravenous administration is administered and the first symptoms of recurring symptoms appear, waiting for another hospitalization to receive the medication is very stressful for the patient. It's impossible to show up at the hospital running the program on request, because the hospitalization date is strictly limited," notes Ernest Syska.
Responsible as a patientAs Dr. Anna Potulska-Chromik emphasizes, the B.67 drug program regarding the use of subcutaneous immunoglobulins is quite demanding for both doctors and patients, who must be highly aware and responsible in conducting treatment according to the arrangements with the doctor.
"Therefore, the patient must undergo several visits, including hospitalization, during which they or their family learn how to administer the medication. Once we determine that the patient has mastered the medication and understands the instructions, we can consider providing them with a 12-week supply," explains the specialist.
As Lucyna Wierzowiecka, president of axON, says, patients thank the ministry and are counting on further systemic changes, including those that will allow more patients to qualify for the B.67 drug program.
Copyrighted material - reprint rules are specified in the regulations .
rynekzdrowia